Crossover study Wikipedia
Table Of Content
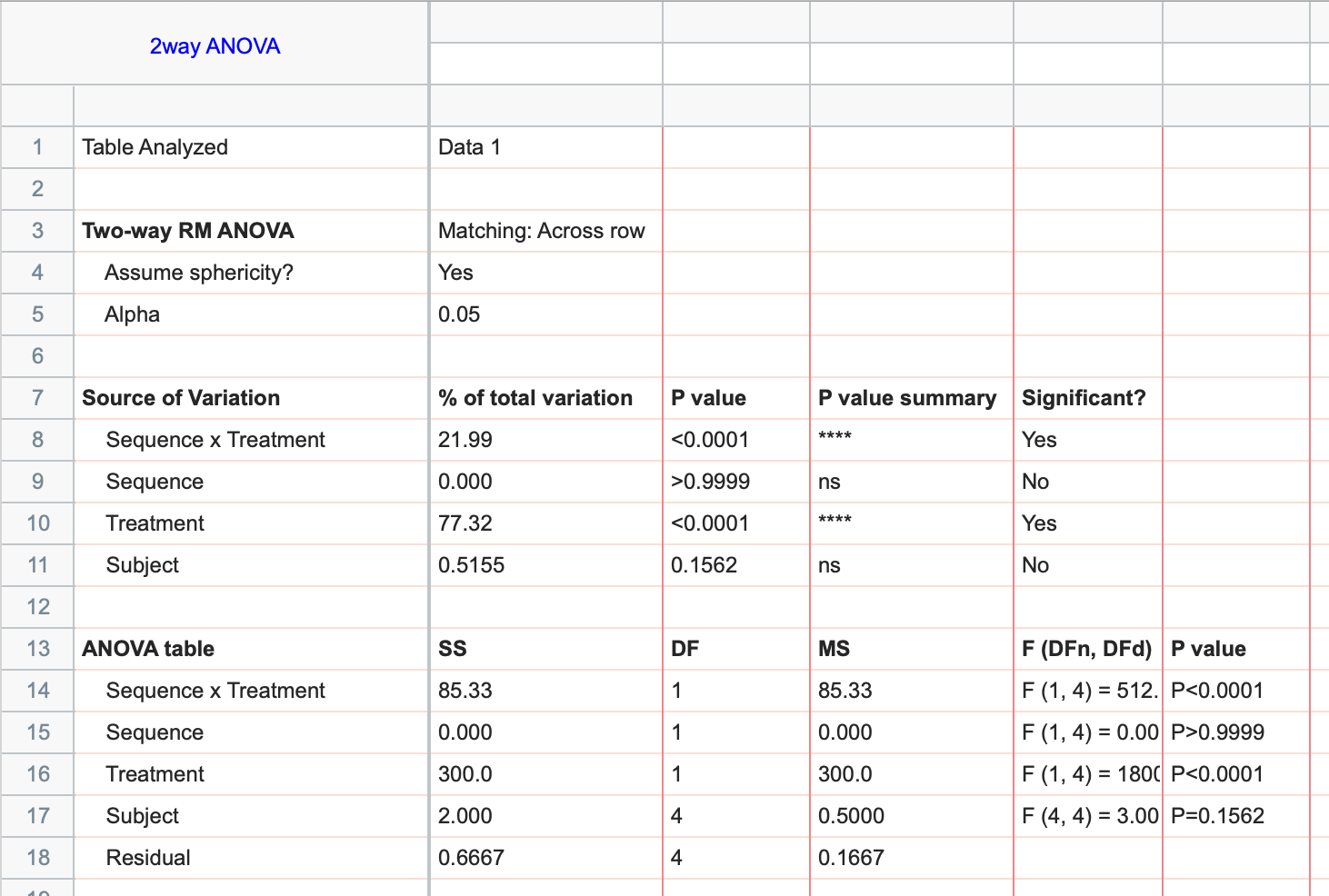
There is a clear need for minimum standards for transparent reporting of crossover trials. The primary advantage of the crossover design is that each subject serves as his or her own control, providing data in each treatment arm of the study. Second is the issue of "carry-over" between treatments, which confounds the estimates of the treatment effects. In practice, "carry-over" effects can be avoided with a sufficiently long "wash-out" period between treatments. However, planning for sufficiently long wash-out periods requires expert knowledge of the dynamics of the treatment, which is often unknown. First is the issue of "order" effects, because it is possible that the order in which treatments are administered may affect the outcome.
What is the value of the resistor in the Zobel circuit if the speaker's resistance is 8 Ω?
The primary objective of this study is to use a case-crossover design to determine if there is a difference in STIs in the periods before and after PrEP for each subject analyzed. The simplest case is where you only have 2 treatments and you want to give each subject both treatments. Certain considerations that are relevant to the crossover design, but play no role in standard parallel-group trials, must receive adequate attention in trial planning and data analysis for the results to be of scientific value. Nearly all crossover are designed to have "balance", whereby all subjects receive the same number of treatments and participate for the same number of periods. In most crossover trials each subject receives all treatments, in a random order.
1.1 Delayed crossover design (Wait list controls)
The suitability of a cross-over design, and the anticipated results, depend crucially on the nature of the intervention. A key question is whether or not the intervention is intended to have long-term effects that persist beyond the intervention phase. For most behavioural interventions, including those administered by speech-and-language therapists, educators and allied health professionals, the goal is to bring about long-term change. Exceptions would be communication aids such as auditory feedback masking, which decreases stuttering while the masker is switched on, but does not produce long-term change (Block et al., 1996). In this regard, most behavioural interventions are unlike pharmaceutical trials, which often focus on the ability of specific drugs to provide symptomatic relief.
Similar articles
An example is when a pharmaceutical treatment causes permanent liver damage so that the patients metabolize future drugs differently. Another example occurs if the treatments are different types of educational tests. Then subjects may be affected permanently by what they learned during the first period.
How Do We Analyze Carryover Effect?
A lab technician then swabs the throat to test for gonorrhea and takes a blood sample to test for syphilis, HIV, and renal function. The blood sample is also used to test for HIV antibodies through an HIV rapid test. Individuals who test HIV antibody negative have a nucleic acid amplification test (NAAT) which is sent for acute HIV screening. Individuals who are positive for any STI except HIV are called back for treatment typically within two to seven days. Individuals who test positive for HIV are taken off PrEP and immediately linked to HIV care by an HIV linkage-to-care specialist.
11 - Analysis - More Complex Designs
Transcutaneous cervical vagus nerve stimulation improves sensory performance in humans: a randomized controlled ... - Nature.com
Transcutaneous cervical vagus nerve stimulation improves sensory performance in humans: a randomized controlled ....
Posted: Sat, 17 Feb 2024 08:00:00 GMT [source]
At the moment, however, we focus on differences in estimated treatment means in two-period, two-treatment designs. The expectation of the treatment mean difference indicates that it is aliased with second-order carryover effects. The order of treatment administration in a crossover experiment is called a sequence and the time of a treatment administration is called a period. Typically, the treatments are designated with capital letters, such as A, B, etc.
Data analysis
The crossover design has a long history in the planning of scientific trials ([1], sect. 1.4) and forms the basis of a large number of clinical studies year after year. Trials in almost all clinical disciplines use the crossover design, but it accounts for a particularly high proportion of studies in the “CNS specialties”—neurology and psychiatry—and of trials on pain treatment. One example of the latter is the frequently cited study of the analgesic effect of synthetic cannabinoids (2). This was a classic crossover trial involving a total of 21 patients with chronic neuropathic pain.
Now when music plays through the speaker, each range of frequencies has the same sound level, with minimal distortion. The total harmonic distortion calculator can tell you more about how this phenomenon can affect sound accuracy. The CONSORT statement aims at comprehensive and complete reporting of randomized controlled trials. This blog introduces you to the statement and why it is an important tool in the research world. Crossover trials are trials in which participants do not only receive one intervention, but multiple, and the effect of the interventions are measured on the same individuals. The probability of a split between treatment A and treatment B preferences under the null hypothesis is equivalent to the odds ratio for the treatment A preference to the treatment B preference being 1.0.
Abbreviations
Short-term exposure to ozone may trigger the onset of Kawasaki disease: An individual-level, case-crossover study in ... - ScienceDirect.com
Short-term exposure to ozone may trigger the onset of Kawasaki disease: An individual-level, case-crossover study in ....
Posted: Wed, 29 Nov 2023 15:10:16 GMT [source]
This form of balance is denoted balanced for carryover (or residual) effects. This example easily explains the main steps that are followed during a crossover trial of the AB/BA type. At first, participants of one group will receive medication A and after a wash-out period, participants of the same group will receive medication B. In principle, the procedure needed for calculation of power and sample sizes for a crossover trial is the same as that which is familiar from the t-test for unpaired samples (18).
When analyzing any STI as an outcome, there was a significant overall increase in STIs between periods. To the best of our knowledge, this is the first study to compare each patient’s STI testing results before and after PrEP initiation to determine if there was a true change in STIs. To do a crossover design, each subject receives each treatment at one time in some order. So, one of its benefits is that you can use each subject as its own control, either as a paired experiment or as a randomized block experiment, the subject serves as a block factor. The smallest crossover design which allows you to have each treatment occurring in each period would be a single Latin square. A crossover study has two advantages over both a parallel study and a non-crossover longitudinal study.

Built on a strong foundation of manufacturing trucks since 1902, GMC now sells in a dozen countries across the world. We do not have observations in all combinations of rows, columns, and treatments since the design is based on the Latin square. She recruits 120 participants into her study and randomly allocates them into 60 participants in group A and 60 participants in group B. At first, participants in group A will receive Valsartan for two weeks in order to treat their high blood pressure, and group B will receive Iosartan for a period of two weeks. After that, there will be a wash-out period of 4 weeks in which the study participants will not receive Iosartan or Valsartan.
Then, you can use the speaker box calculator to aid in the creation of the housing of your components. However, it still allows signals to go to the wrong speaker (due to the low filter slope value), so the damage could yet be done to the tweeter if it receives a significant signal with a lower frequency than it can handle. However, there is a problem when it comes to connecting our multiple speaker solution to an amplifier. The speaker cable contains all frequencies (as electronic signals), so the woofer will still get the high frequencies, and the tweeter the low frequencies. This frequency mismatch will produce sound distortion, and could even damage a speaker if it gets a loud enough signal at the wrong frequency. That means low volume and sound distortions at low frequencies, such as the bass instrument in a music track.
Obviously, randomization is very important if the crossover design is not uniform within sequences because the underlying assumption is that the sequence effect is negligible. Randomization is important in crossover trials even if the design is uniform within sequences because biases could result from investigators assigning patients to treatment sequences. Although we included all STI testing visits up to 365 days after PrEP initiation, a full 365-day period was not available for all patients included in the analysis since the analysis period ended in May 2017. For example, an individual who began PrEP in October 2016 would only have contributed eight months to the after-PrEP period. However, our protocols require patients to return at three month intervals to refill their PrEP prescription, and a majority of individuals included had at least two STI testing visits in the after-PrEP period. Furthermore, we adjusted for time under observation by fitting incidence models.
If information about funding sources and number of study sites was unclear from the trial report, we requested clarification from the trialists. With liberal definitions of adequacy [12], the reporting of patient preference and methods of random sequence generation, and allocation concealment were recorded. We also noted the handling of non-compliers, carryover, period, and treatment effects. We calculated descriptive summary statistics both overall and stratified by study design. We entered the data into an electronic database such that duplicate entries existed for each study; when two entries did not match, we reached consensus through discussion and 3rd party arbitration (BR). To exploit these advantages to the full, a few specific pitfalls must be avoided in the planning and analysis of crossover trials.
Comments
Post a Comment