Design, analysis, and presentation of crossover trials PMC
Table Of Content

Statistics.com is powered by Elder Research, a data science consultancy with 25 years of experience in data analytics, and is certified to operate by the State Council of Higher Education for Virginia (SCHEV). By submitting your information, you agree to receive email communications from Statistics.com. 6)In the example of this article, the carryover effect is evaluated through sequence effect analysis. 3)In addition to the independent variables, a variable that the researcher wants to control as a factor that can affect the dependent variable is designated a covariate. This is a Case 2 where the column factor, the cows are nested within the square, but the row factor, period, is the same across squares. Use the viewlet below to walk through an initial analysis of the data (cow_diets.mwx | cow_diets.csv) for this experiment with cow diets.
Analysis of the data from a 2x2 crossover for a binary outcome, assuming null period effects
Another example occurs in bioequivalence trials where some researchers argue that carryover effects should be null. This is because blood concentration levels of the drug or active ingredient are monitored and any residual drug administered from an earlier period would be detected. A carryover effect is defined as the effect of the treatment from the previous time period on the response at the current time period. Randomization is performed to eliminate selection bias and to provide statistical evidence for quantitative evaluation. In parallel design, randomization to different treatment groups (A or B) can ensure independence between groups. However, in the crossover design, randomization is performed in sequence, that is, AB or BA sequence, so that virtually no independence between treatment groups is guaranteed.
Correct procedure for statistical analysis
Second, optimal crossover designs are statistically efficient, and so require fewer subjects than do non-crossover designs (even other repeated measures designs). Test and reference formulations were studied in a bioequivalence trial that used a 2 × 2 crossover design. There were 28 healthy volunteers, (instead of patients with disease), who were randomized (14 each to the TR and RT sequences).
Analysis of variance table to test other effects
Actor Grant Gustin, who portrayed the speedster, commemorated the occasion with a throwback photo of himself in a Flash bathrobe from April 25, 2016. He looked for a photo from the set on every April 25 during the nine years they were in production, he wrote, but the cheeky shot was “the only Flash related April 25th photo” he had. The ominous scene set up multiple important storylines in the nine-season series, from villain Reverse-Flash looming large in the first season to a massive DC Comics crossover storyline that didn’t come into play until Season 6.
Complex Carryover
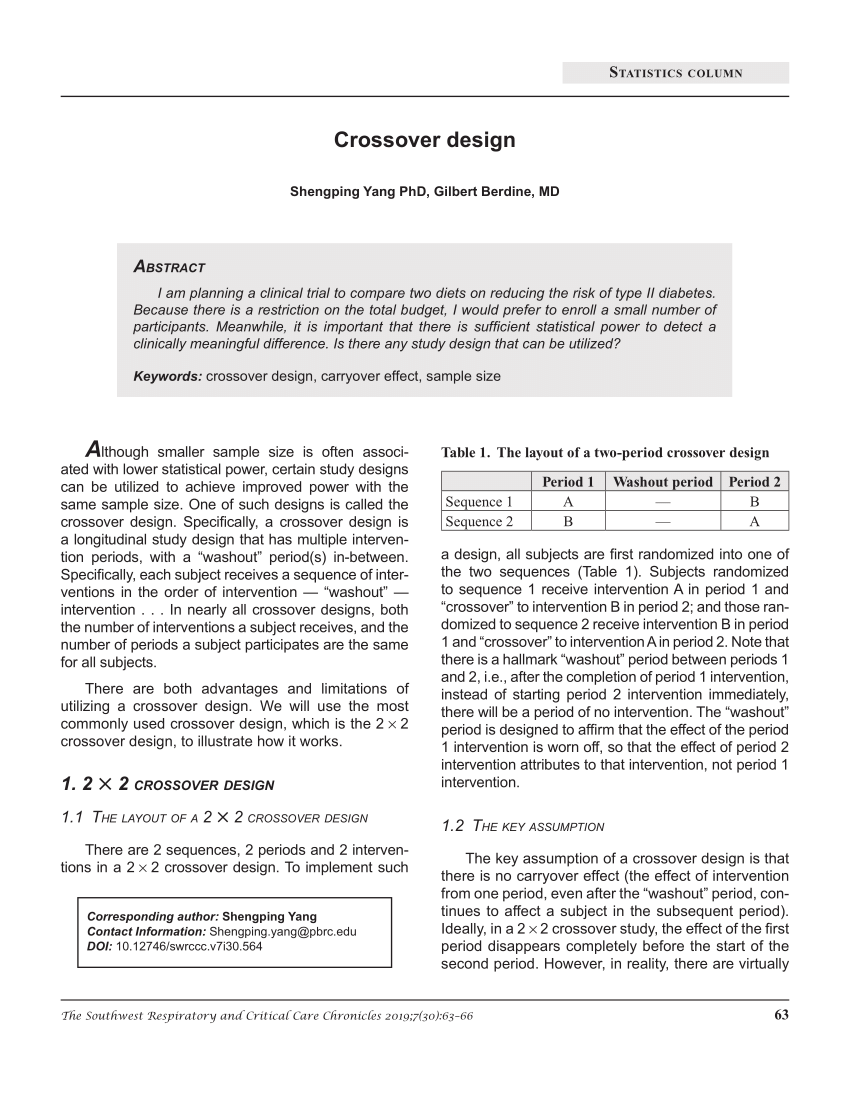
Subjects in a crossover design study are assigned to receive two or more treatments in a particular sequence. Imagine a study in which some subjects receive Treatment A on a given day (the first period) and a week later receive Treatment B (the second period). Others subjects (usually close to an equal number) would receive Treatment B first and then, 1 week later, receive Treatment A. Such a study would be described as having a two-period, two-treatment, two-sequence crossover design. Crossover designs can involve various numbers of treatments, sequences, and periods.
Balanced Designs
Therefore, as discussed above, it is necessary to test whether the treatment effect shown in the first period remains in the second period. The standard 2 × 2 crossover design is used to assess between two groups (test group A and control group B). Each subject is randomly allocated to either an AB sequence or a BA sequence. Subjects in the AB sequence receive treatment A at the first period and treatment B at the second period.
Examining acute psychopharmacological effects of nicotine vaping versus heated tobacco products in a randomised ... - Nature.com
Examining acute psychopharmacological effects of nicotine vaping versus heated tobacco products in a randomised ....
Posted: Tue, 19 Dec 2023 08:00:00 GMT [source]
The two trial periods in which the patient receives the different treatments whose effects are being compared must be separated by a washout phase that is sufficiently long to rule out any carryover effect. In other words, the effect of the first treatment must have disappeared completely before the beginning of the second period. Researchers analyzing the data of crossover trials often proceed as though they were performing a simple pre/post comparison. Unfortunately this error can be observed time and time again, even in renowned journals (3– 8). Crossover trials in which the paired t-test (or any other procedure for paired samples) was used for analysis are methodologically flawed and do not contribute to evidence-based evaluation of the treatments concerned. From [16], the direct treatment effects are aliased with the sequence effect and the carryover effects, whereas the treatment difference only is aliased with the sequence effect.
Associated Data
Caffeine supplementation improves the cognitive abilities and shooting performance of elite e-sports players: a ... - Nature.com
Caffeine supplementation improves the cognitive abilities and shooting performance of elite e-sports players: a ....
Posted: Wed, 24 Jan 2024 08:00:00 GMT [source]
For the study of new and developmental drugs, crossover studies are extremely popular [4,5], particularly when the new treatment may only be a slight modification to the standard. In this case, there is likely to be a positive correlation in the responses to the new and old treatments making the crossover design ideal [6]. Crossover studies are most appropriate in studies where the effects of the treatment(s) are short-lived and reversible and are best suited to trials related to symptomatic but chronic conditions or diseases [3,7]. It is generally agreed that the crossover design should not be used when the condition of interest is unstable and may change regardless of interventions [3]. In spite of criticism [8], however, the crossover design appears to be used commonly in inappropriate circumstances [3,9].
In these designs, individual subjects are randomized to treatment sequences (as opposed to treatment groups as occurs in parallel groups study designs). The described confirmatory procedures based on unpaired t-statistics assume (approximate) normality of the distributions to be analyzed. Not infrequently, however, only a weaker model assumption seems realistic, according to which the variables under analysis have distributions of some unspecified form being common to both sequence groups. The medians of these distributions are assumed to decompose into a sum of terms representing the respective effects of treatment and period, as well as possible carryover effects. A strategy for confirmatory analysis whose validity is granted under these weaker conditions consists in replacing two-sample t-tests with Wilcoxon rank sum tests (20) throughout.
In a trial involving pharmaceutical products, the length of the washout period usually is determined as some multiple of the half-life of the pharmaceutical product within the population of interest. For example, an investigator might implement a washout period equivalent to 5 (or more) times the length of the half-life of the drug concentration in the blood. The period effect implies that the effect of the same treatment received at two different periods is different for each period and corresponds to Pj in Equation (1).
In other words, it is difficult to analyze the carryover effect in the simplified 2 × 2 crossover design [5]; therefore, it is important in the study planning stage to design such that the carryover effect does not occur. For example, there is a method to set a sufficient washout period until the treatment effect or change disappears. In the case of drug studies, a washout period is sometimes set at 3–4 times or more of the blood plasma elimination half-life. Under the usual statistical model assumptions for the parametric analysis of crossover trials (19), this question can be answered by means of the approximate equation shown in Box 3b. Since the variance due to measurement error is generally smaller than that which can be ascribed to between-subject variability, the difference is very often substantial. In a situation where the between-subject variance is twice as large as that due to measurement error, for instance, six times as many patients are required to achieve the same power in a parallel-group study as in a crossover trial.
During the design phase of a trial, the question may arise as to which crossover design provides the best precision. For our purposes, we label one design as more precise than another if it yields a smaller variance for the estimated treatment mean difference. The rationale for this is that the previously administered treatment is “washed out” of the patient and, therefore, it can not affect the measurements taken during the current period. This may be true, but it is possible that the previously administered treatment may have altered the patient in some manner so that the patient will react differently to any treatment administered from that time onward.
The teaser shows off the Terrain AT4’s redesigned front fascia, illustrating the compact crossover’s bolder, more assertive design. During this time, the performance of our website may be affected - searches may run slowly and some pages may be temporarily unavailable. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
Our study is nested within a larger analysis of RCTs [10] where we used an extended version of the Cochrane search strategy (phase 1) to identify all randomized trials published in December 2000 and indexed on PubMed by July 2002 [11]. A randomized trial was defined as a prospective study assessing health-care interventions in human participants who were randomly allocated to study groups. Abstracts were initially screened to exclude obvious non-trials, and complete primary reports in the languages AWC could read (English and French) were reviewed for all remaining studies.
Further studies in other jurisdictions should replicate these analyses to determine if our finding of an increase in rectal chlamydia is an aberration or an actual trend. A recent survey of physicians that asked about their willingness to prescribe PrEP reported that 96% of respondents noted concerns about patient adherence to the regimen (18). Pre-exposure prophylaxis (PrEP) is an effective method for reducing HIV incidence among at-risk populations. However, concerns exist over the potential for an increase in sexually transmitted infections (STIs) following PrEP initiation. The objective of this study is to compare the STI incidence before and after PrEP initiation within subjects among a cohort of men who have sex with men (MSM) in Los Angeles, California. If you look at how we have coded data here, we have another column called residual treatment.
Comments
Post a Comment